From The Fu Foundation School of Engineering and Applied Science
At
3.1.24
Holly Evarts
212-854-3206
holly.evarts@columbia.edu
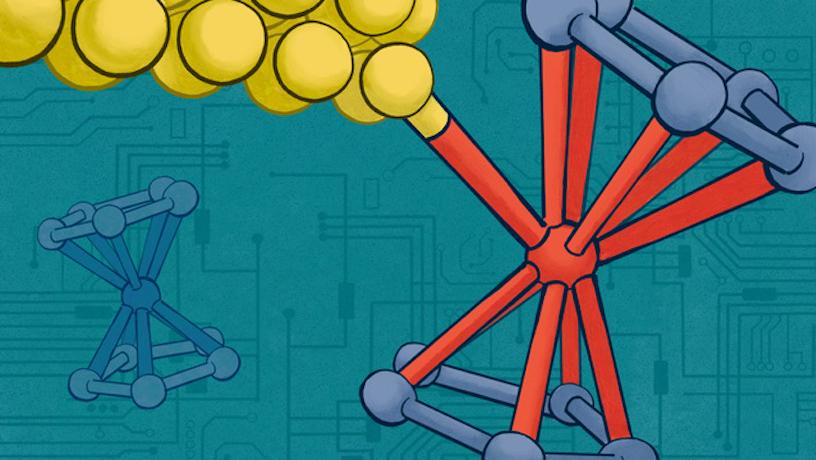
Photo Credit: Venkatraman lab
In a new Nature Communications study, Columbia Engineering researchers report that they have built highly conductive, tunable single-molecule devices in which the molecule is attached to leads by using direct metal-metal contacts.
See the science paper for instructive material with images.
Their novel approach uses light to control the electronic properties of the devices and opens the door to broader use of metal-metal contacts that could facilitate electron transport across the single-molecule device.
The challenge
As devices continue to shrink, their electronic components must also be miniaturized. Single-molecule devices, which use organic molecules as their conductive channels, have the potential to resolve the miniaturization and functionalization challenges faced by traditional semiconductors. Such devices offer the exciting possibility of being controlled externally by using light, but — until now – researchers have not been able to demonstrate this.
“With this work, we’ve unlocked a new dimension in molecular electronics, where light can be used to control how a molecule binds within the gap between two metal electrodes,” said Latha Venkataraman, a pioneer in molecular electronics and Lawrence Gussman Professor of Applied Physics and professor of chemistry at Columbia Engineering. “It’s like flipping a switch at the nanoscale, opening up all kinds of possibilities for designing smarter and more efficient electronic components.”
The approach
Venkataraman’s group has been studying the fundamental properties of single-molecule devices for almost two decades, exploring the interplay of physics, chemistry, and engineering at the nanometer scale. Her underlying focus is on building single-molecule circuits, a molecule attached to two electrodes, with varied functionality, where the circuit structure is defined with atomic precision.
Her group, as well as those creating functional devices with graphene, a carbon-based two-dimensional material, have known that making good electrical contacts between metal electrodes and carbon systems is a major challenge. One solution would be to use organo-metallic molecules and devise methods to interface electrical leads to the metal atoms within the molecule. Towards this goal, they decided to explore the use of organo-metallic iron-containing ferrocene molecules, which are also considered to be tiny building blocks in the world of nanotechnology. Just like LEGO pieces can be stacked together to create complex structures, ferrocene molecules can be used as building blocks to construct ultra-small electronic devices. The team used a molecule terminated by a ferrocene group comprising two carbon-based cyclopentadienyl rings that sandwich an iron atom. They then used light to leverage the electrochemical properties of the ferrocene-based molecules to form a direct bond between the ferrocene iron center and the gold (Au) electrode when the molecule was in an oxidized state (i.e. when the iron atom had lost one electron). In this state, they discovered that ferrocene could bind to the gold electrodes used to connect the molecule to the external circuitry. Technically, oxidizing the ferrocene enabled the binding of a Au0 to an Fe3+ center.
“By harnessing the light-induced oxidation, we found a way to manipulate these tiny building blocks at room temperature, opening doors to a future where light can be used to control the behavior of electronic devices at the molecular level,” said the study’s lead author Woojung Lee, who is a PhD student in Venkararaman’s lab.
Potential impact
Venkataraman’s new approach will enable her team to extend the types of molecular terminations (contact) chemistries they can use for creating single-molecule devices. This study also shows the ability to turn on and off this contact by using light to change the oxidation state of the ferrocene, demonstrating a light-switchable ferrocene-based single-molecule device. The light-controlled devices could pave the way for the development of sensors and switches that respond to specific light wavelengths, offering more versatile and efficient components for a wide range of technologies.
The team
This work was a collaborative effort involving synthesis, measurements, and calculations. The synthesis was done primarily at Columbia by Michael Inkpen, who was a post-doc in the Venkataraman group and is now an assistant professor at the University of Southern California. All the measurements were made by Woojung Lee, a graduate student in the Venkataraman group. The calculations were performed both by graduate students in the Venkataraman group and by collaborators from the University of Regensburg in Germany.
What’s next
The researchers are now exploring the practical applications of light-controlled single-molecule devices. This could include optimizing device performance, studying their behavior under different environmental conditions, and refining additional functionalities enabled by the metal-metal interface.
See the full article here .
Comments are invited and will be appreciated, especially if the reader finds any errors which I can correct.
five-ways-keep-your-child-safe-school-shootings
Please help promote STEM in your local schools.
The Columbia University Fu Foundation School of Engineering and Applied Science is the engineering and applied science school of Columbia University. It was founded as the School of Mines in 1863 and then the School of Mines, Engineering and Chemistry before becoming the School of Engineering and Applied Science. On October 1, 1997, the school was renamed in honor of Chinese businessman Z.Y. Fu, who had donated $26 million to the school.
The Fu Foundation School of Engineering and Applied Science maintains a close research tie with other institutions including National Aeronautics and Space Administration, IBM, Massachusetts Institute of Technology, and The Earth Institute. Patents owned by the school generate over $100 million annually for the university. Faculty and alumni are responsible for technological achievements including the developments of FM radio and the maser.
The School’s applied mathematics, biomedical engineering, computer science and the financial engineering program in operations research are very famous and ranked high. The current faculty include members of the National Academy of Engineering and Nobel laureats. In all, the faculty and alumni of Columbia Engineering have won a number of Nobel Prizes in physics, chemistry, medicine, and economics.
The school consists of approximately 300 undergraduates in each graduating class and maintains close links with its undergraduate liberal arts sister school Columbia College which shares housing with SEAS students.
Original charter of 1754
Included in the original charter for Columbia College was the direction to teach “the arts of Number and Measuring, of Surveying and Navigation […] the knowledge of […] various kinds of Meteors, Stones, Mines and Minerals, Plants and Animals, and everything useful for the Comfort, the Convenience and Elegance of Life.” Engineering has always been a part of Columbia, even before the establishment of any separate school of engineering.
An early and influential graduate from the school was John Stevens, Class of 1768. Instrumental in the establishment of U.S. patent law. Stevens procured many patents in early steamboat technology; operated the first steam ferry between New York and New Jersey; received the first railroad charter in the U.S.; built a pioneer locomotive; and amassed a fortune, which allowed his sons to found the Stevens Institute of Technology.
When Columbia University first resided on Wall Street, engineering did not have a school under the Columbia umbrella. After Columbia outgrew its space on Wall Street, it relocated to what is now Midtown Manhattan in 1857. Then President Barnard and the Trustees of the University, with the urging of Professor Thomas Egleston and General Vinton, approved the School of Mines in 1863. The intention was to establish a School of Mines and Metallurgy with a three-year program open to professionally motivated students with or without prior undergraduate training. It was officially founded in 1864 under the leadership of its first dean, Columbia professor Charles F. Chandler, and specialized in mining and mineralogical engineering. An example of work from a student at the School of Mines was William Barclay Parsons, Class of 1882. He was an engineer on the Chinese railway and the Cape Cod and Panama Canals. Most importantly he worked for New York, as a chief engineer of the city’s first subway system, the Interborough Rapid Transit Company. Opened in 1904, the subway’s electric cars took passengers from City Hall to Brooklyn, the Bronx, and the newly renamed and relocated Columbia University in Morningside Heights, its present location on the Upper West Side of Manhattan.
Columbia University was founded in 1754 as King’s College by royal charter of King George II of England. It is the oldest institution of higher learning in the state of New York and the fifth oldest in the United States.
University Mission Statement
Columbia University is one of the world’s most important centers of research and at the same time a distinctive and distinguished learning environment for undergraduates and graduate students in many scholarly and professional fields. The University recognizes the importance of its location in New York City and seeks to link its research and teaching to the vast resources of a great metropolis. It seeks to attract a diverse and international faculty and student body, to support research and teaching on global issues, and to create academic relationships with many countries and regions. It expects all areas of the University to advance knowledge and learning at the highest level and to convey the products of its efforts to the world.
Columbia University is a private Ivy League research university in New York City. Established in 1754 on the grounds of Trinity Church in Manhattan Columbia is the oldest institution of higher education in New York and the fifth-oldest institution of higher learning in the United States. It is one of nine colonial colleges founded prior to the Declaration of Independence, seven of which belong to the Ivy League. Columbia is ranked among the top universities in the world by major education publications.
Columbia was established as King’s College by royal charter from King George II of Great Britain in reaction to the founding of Princeton College. It was renamed Columbia College in 1784 following the American Revolution, and in 1787 was placed under a private board of trustees headed by former students Alexander Hamilton and John Jay. In 1896, the campus was moved to its current location in Morningside Heights and renamed Columbia University.
Columbia scientists and scholars have played an important role in scientific breakthroughs including brain-computer interface; the laser and maser; nuclear magnetic resonance; the first nuclear pile; the first nuclear fission reaction in the Americas; the first evidence for plate tectonics and continental drift; and much of the initial research and planning for the Manhattan Project during World War II. Columbia is organized into twenty schools, including four undergraduate schools and 15 graduate schools. The university’s research efforts include the Lamont–Doherty Earth Observatory, the Goddard Institute for Space Studies, and accelerator laboratories with major technology firms such as IBM. Columbia is a founding member of the Association of American Universities and was the first school in the United States to grant the M.D. degree. With over 14 million volumes, Columbia University Library is the third largest private research library in the United States.
The university’s endowment stands among the largest of any academic institution. Columbia’s alumni, faculty, and staff have included: Founding Fathers of the United States—among them a co-author of the United States Constitution and a co-author of the Declaration of Independence; U.S. presidents; foreign heads of state; justices of the United States Supreme Court; Nobel laureates; Fields Medalists; many members of National Academy of Sciences; living billionaires; Olympic medalists; Academy Award winners; and Pulitzer Prize recipients.




