From The DOE’s Lawrence Berkeley National Laboratory
6.19.24
Aliyah Kovner
AKovner@lbl.gov
An approach developed by materials scientists is already yielding discoveries that could improve the efficiency and durability of metallic catalysts used in a variety of processes.
Senior author Haimei Zheng, left, and first author Qiubo Zhang look at the results of measurements obtained using their new technology, which pairs with powerful microscopes at Berkeley Lab’s National Center for Electron Microscopy. Credit: Thor Swift/Berkeley Lab
A team led by Lawrence Berkeley National Laboratory (Berkeley Lab) has invented a technique to study electrochemical processes at the atomic level with unprecedented resolution and used it to gain new insights into a popular catalyst material. Electrochemical reactions – chemical transformations that are caused by or accompanied by the flow of electric currents – are the basis of batteries, fuel cells, electrolysis, and solar-powered fuel generation, among other technologies. They also drive biological processes such as photosynthesis and occur under the Earth’s surface in the formation and breakdown of metal ores.
The scientists have developed a cell – a small enclosed chamber that can hold all the components of an electrochemical reaction – that can be paired with transmission electron microscopy (TEM) to generate precise views of a reaction at atomic scale. Better yet, their device, which they call a polymer liquid cell (PLC), can be frozen to stop the reaction at specific timepoints, so scientists can observe composition changes at each stage of a reaction with other characterization tools. In a paper published June 19 in Nature, the team describes their cell and a proof of principle investigation using it to study a copper catalyst that reduces carbon dioxide to generate fuels.
“This is a very exciting technical breakthrough that shows something we could not do before is now possible. The liquid cell allows us to see what’s going on at the solid-liquid interface during reactions in real time, which are very complex phenomena. We can see how the catalyst surface atoms move and transform into different transient structures when interacting with the liquid electrolyte during electrocatalytic reactions,” said Haimei Zheng, lead author and senior scientist in Berkeley Lab’s Materials Science Division.
“It’s very important for catalyst design to see how a catalyst works and also how it degrades. If we don’t know how it fails, we won’t be able to improve the design. And we’re very confident we’re going to see that happen with this technology,” said co-first author Qiubo Zhang, a postdoctoral research fellow in Zheng’s lab.
Zheng and her colleagues are excited to use the PLC on a variety of other electrocatalytic materials, and have already begun investigations into problems in lithium and zinc batteries. The team is optimistic that details revealed by the PLC-enabled TEM could lead to improvements in all electrochemical-driven technologies.
New insights into a popular catalyst
The scientists tested the PLC approach on a copper catalyst system that is a hot subject of research and development because it can transform atmospheric carbon dioxide molecules into valuable carbon-based chemicals such as methanol, ethanol, and acetone. However, a deeper understanding of copper-based CO2 reducing catalysts is needed to engineer systems that are durable and efficiently produce a desired carbon product rather than off-target products.
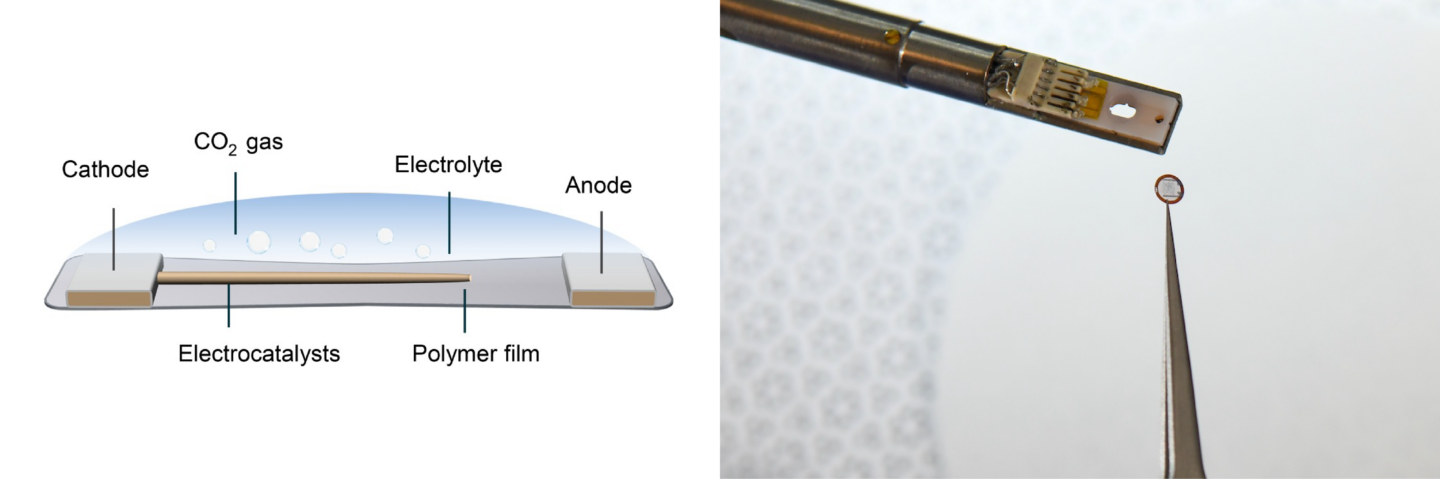
On the left, a schematic showing the different components of the polymer liquid cell (PLC) that the team developed. On the right, a photograph of the PLC (the small circle held in the tweezers) next to the holding device that supports the PLC and inserts it into the transmission electron microscope. (Credit: Berkeley Lab, Thor Swift/Berkeley Lab)
Zheng’s team used the powerful microscopes at the National Center for Electron Microscopy, part of Berkeley Lab’s Molecular Foundry [below], to study the area within the reaction called the solid-liquid interface, where the solid catalyst that has electrical current through it meets the liquid electrolyte. The catalyst system they put inside the cell consists of solid copper with an electrolyte of potassium bicarbonate (KHCO3) in water. The cell is composed of platinum, aluminum oxide, and a super thin, 10 nanometer polymer film.
Using electron microscopy, electron energy loss spectroscopy, and energy-dispersive X-ray spectroscopy, the researchers captured unprecedented images and data that revealed unexpected transformations at the solid-liquid interface during the reaction. The team observed copper atoms leaving the solid, crystalline metal phase and mingling with carbon, hydrogen, and oxygen atoms from the electrolyte and CO2 to form a fluctuating, amorphous state between the surface and the electrolyte, which they dubbed an “amorphous interphase” because it is neither solid nor liquid. This amorphous interphase disappears again when the current stops flowing, and most of the copper atoms return to the solid lattice.
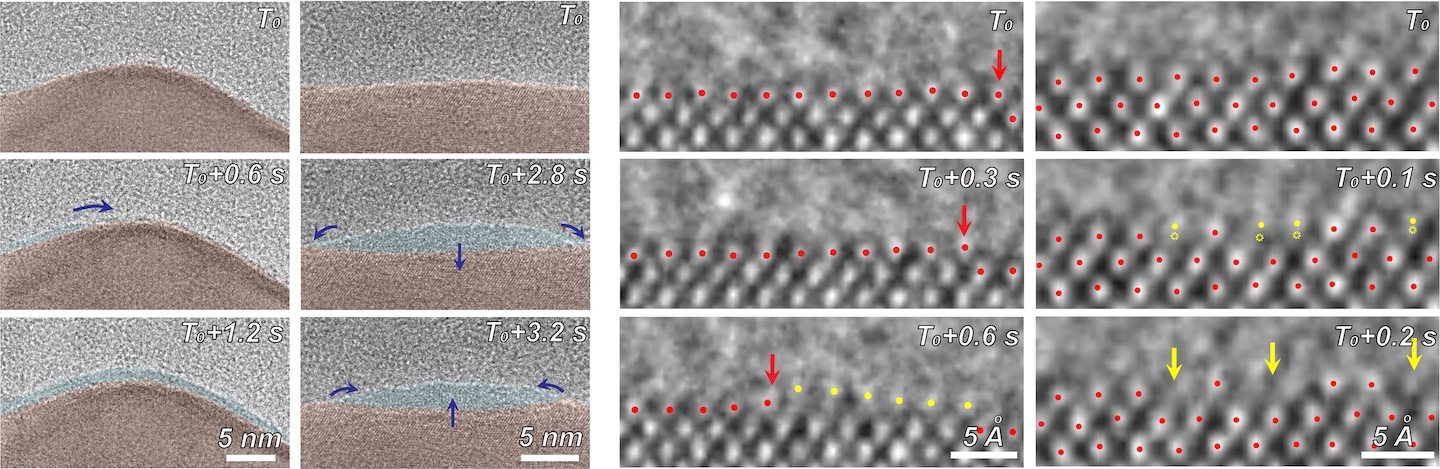
(Left) In-situ TEM images showing the appearance, movement, and disappearance of the amorphous interphase (light blue) between the solid copper catalyst (brown) and liquid electrolyte (grey) at different timepoints. The dark blue arrows indicate directions of the amorphous phase movement. (Right) High-resolution TEM images showing a magnified view of the catalyst atomic dynamics mediated by the amorphous interphase during the carbon dioxide electroreduction reaction. Red dots highlight copper atoms on solid copper catalyst surface. Yellow dots highlight copper atoms with displacements while transforming into the amorphous phase. Red arrows indicate the atomic step. Yellow arrows indicate the resulting atomic roughness. (Credit: Zheng et al/Berkeley Lab)
According to Zhang, the dynamics of the amorphous interphase could be leveraged in the future to make the catalyst more selective for specific carbon products. Additionally, understanding the interphase will help scientists combat degradation – which occurs on the surface of all catalysts over time – to develop systems with longer operational lifetimes.
“Previously, people relied on the initial surface structure to design the catalyst for both efficiency and stability. The discovery of the amorphous interphase challenges our previous understanding of solid-liquid interfaces, prompting a need to consider its effects when devising strategies,” said Zhang.
“During the reaction, the structure of the amorphous interphase changes continuously, impacting performance. Studying the dynamics of the solid-liquid interface can aid in understanding these changes, allowing for the development of suitable strategies to enhance catalyst performance,” added Zhigang Song, co-first author and postdoctoral scholar at Harvard University.
The other authors on this work were Xianhu Sun, Yang Liu, Jiawei Wan, Sophia. B. Betzler, Qi Zheng, Junyi Shangguan, Karen. C. Bustillo, Peter Ercius, Prineha Narang, and Yu Huang. Funding was provided by the U.S. Department of Energy (DOE) Office of Science. The Molecular Foundry is a DOE Office of Science user facility.
See the full article here .
Comments are invited and will be appreciated, especially if the reader finds any errors which I can correct.
five-ways-keep-your-child-safe-school-shootings
Please help promote STEM in your local schools.


Bringing Science Solutions to the World
In the world of science, The Lawrence Berkeley National Laboratory (Berkeley Lab) is synonymous with “excellence.” A number of Nobel prizes are associated with Berkeley Lab. Lab scientists are members of the The National Academy of Sciences, one of the highest honors for a scientist in the United States. A number of our scientists have won the National Medal of Science, our nation’s highest award for lifetime achievement in fields of scientific research. A number of our engineers have been elected to the The National Academy of Engineering, and a number of our scientists have been elected into The Institute of Medicine. In addition, Berkeley Lab has trained thousands of university science and engineering students who are advancing technological innovations across the nation and around the world.
Berkeley Lab is a member of the national laboratory system supported by The DOE through its Office of Science. It is managed by the University of California-Berkeley and is charged with conducting unclassified research across a wide range of scientific disciplines. Located on a 202-acre site in the hills above The University of California-Berkeley campus that offers spectacular views of the San Francisco Bay, Berkeley Lab employs a large number of scientists, engineers and support staff. Technologies developed at Berkeley Lab have generated billions of dollars in revenues, and thousands of jobs. Savings as a result of Berkeley Lab developments in lighting and windows, and other energy-efficient technologies, have also been in the billions of dollars.
Berkeley Lab was founded in 1931 by Ernest Orlando Lawrence, a University of California-Berkeley physicist who won the 1939 Nobel Prize in physics for his invention of the cyclotron, a circular particle accelerator that opened the door to high-energy physics. It was Lawrence’s belief that scientific research is best done through teams of individuals with different fields of expertise, working together. His teamwork concept is a Berkeley Lab legacy that continues today.
History
1931–1941
The laboratory was founded on August 26, 1931, by Ernest Lawrence, as the Radiation Laboratory of the University of California-Berkeley, associated with the Physics Department. It centered physics research around his new instrument, the cyclotron, a type of particle accelerator for which he was awarded the Nobel Prize in Physics in 1939.

LBNL 88 inch cyclotron.
Throughout the 1930s, Lawrence pushed to create larger and larger machines for physics research, courting private philanthropists for funding. He was the first to develop a large team to build big projects to make discoveries in basic research. Eventually these machines grew too large to be held on the university grounds, and in 1940 the lab moved to its current site atop the hill above campus. Part of the team put together during this period includes two other young scientists who went on to establish large laboratories; J. Robert Oppenheimer founded The DOE’s Los Alamos Laboratory, and Robert Wilson founded The DOE’s Fermi National Accelerator Laboratory.
1942–1950
Leslie Groves visited Lawrence’s Radiation Laboratory in late 1942 as he was organizing the Manhattan Project, meeting J. Robert Oppenheimer for the first time. Oppenheimer was tasked with organizing the nuclear bomb development effort and founded today’s DOE Los Alamos National Laboratory to help keep the work secret. At the RadLab, Lawrence and his colleagues developed the technique of electromagnetic enrichment of uranium using their experience with cyclotrons. The “calutrons” (named after the University) became the basic unit of the massive Y-12 facility in Oak Ridge, Tennessee. Lawrence’s lab helped contribute to what have been judged to be the three most valuable technology developments of the war (the atomic bomb, proximity fuse, and radar). The cyclotron, whose construction was stalled during the war, was finished in November 1946. The Manhattan Project shut down two months later.
1951–2018
After the war, the Radiation Laboratory became one of the first laboratories to be incorporated into the Atomic Energy Commission (AEC) (now The Department of Energy . The most highly classified work remained at Los Alamos, but the RadLab remained involved. Edward Teller suggested setting up a second lab similar to Los Alamos to compete with their designs. This led to the creation of an offshoot of the RadLab (now The DOE’s Lawrence Livermore National Laboratory) in 1952. Some of the RadLab’s work was transferred to the new lab, but some classified research continued at Berkeley Lab until the 1970s, when it became a laboratory dedicated only to unclassified scientific research.
Shortly after the death of Lawrence in August 1958, the UC Radiation Laboratory (both branches) was renamed the Lawrence Radiation Laboratory. The Berkeley location became the Lawrence Berkeley Laboratory in 1971, although many continued to call it the RadLab. Gradually, another shortened form came into common usage, LBNL. Its formal name was amended to Ernest Orlando Lawrence Berkeley National Laboratory in 1995, when “National” was added to the names of all DOE labs. “Ernest Orlando” was later dropped to shorten the name. Today, the lab is commonly referred to as “Berkeley Lab”.
The Alvarez Physics Memos are a set of informal working papers of the large group of physicists, engineers, computer programmers, and technicians led by Luis W. Alvarez from the early 1950s until his death in 1988. Over 1700 memos are available on-line, hosted by the Laboratory.
The lab remains owned by the Department of Energy , with management from the University of California-Berkeley. Companies such as Intel were funding the lab’s research into computing chips.
Science mission
From the 1950s through the present, Berkeley Lab has maintained its status as a major international center for physics research, and has also diversified its research program into almost every realm of scientific investigation. Its mission is to solve the most pressing and profound scientific problems facing humanity, conduct basic research for a secure energy future, understand living systems to improve the environment, health, and energy supply, understand matter and energy in the universe, build and safely operate leading scientific facilities for the nation, and train the next generation of scientists and engineers.
The Laboratory’s 20 scientific divisions are organized within six areas of research: Computing Sciences; Physical Sciences; Earth and Environmental Sciences; Biosciences; Energy Sciences; and Energy Technologies. Berkeley Lab has six main science thrusts: advancing integrated fundamental energy science; integrative biological and environmental system science; advanced computing for science impact; discovering the fundamental properties of matter and energy; accelerators for the future; and developing energy technology innovations for a sustainable future. It was Lawrence’s belief that scientific research is best done through teams of individuals with different fields of expertise, working together. His teamwork concept is a Berkeley Lab tradition that continues today.
Berkeley Lab operates five major National User Facilities for the DOE Office of Science:
The Advanced Light Source (ALS) is a synchrotron light source with 41 beam lines providing ultraviolet, soft x-ray, and hard x-ray light to scientific experiments.

The ALS is one of the world’s brightest sources of soft x-rays, which are used to characterize the electronic structure of matter and to reveal microscopic structures with elemental and chemical specificity. About 2,500 scientist-users carry out research at ALS every year. Berkeley Lab is proposing an upgrade of ALS which would increase the coherent flux of soft x-rays by two-three orders of magnitude.
Berkeley Lab Laser Accelerator (BELLA) Center


The DOE Joint Genome Institute supports genomic research in support of the DOE missions in alternative energy, global carbon cycling, and environmental management. The JGI’s partner laboratories are Berkeley Lab, the DOE’s Lawrence Livermore National Laboratory, the DOE’s Oak Ridge National Laboratory (ORNL), the DOE’s Pacific Northwest National Laboratory (PNNL), and the DOE’s HudsonAlpha Institute for Biotechnology . The JGI’s central role is the development of a diversity of large-scale experimental and computational capabilities to link sequence to biological insights relevant to energy and environmental research. A large number of scientist-users take advantage of JGI’s capabilities for their research every year.

The LBNL Molecular Foundry is a multidisciplinary nanoscience research facility. Its seven research facilities focus on Imaging and Manipulation of Nanostructures; Nanofabrication; Theory of Nanostructured Materials; Inorganic Nanostructures; Biological Nanostructures; Organic and Macromolecular Synthesis; and Electron Microscopy. Approximately 700 scientist-users make use of these facilities in their research every year.
The DOE’s NERSC National Energy Research Scientific Computing Center is the scientific computing facility that provides large-scale computing for the DOE’s unclassified research programs. Its current systems provide over 3 billion computational hours annually. NERSC supports 6,000 scientific users from universities, national laboratories, and industry.
DOE’s NERSC National Energy Research Scientific Computing Center at Lawrence Berkeley National Laboratory.


NERSC is a DOE Office of Science User Facility.
The DOE’s Energy Science Network is a high-speed network infrastructure optimized for very large scientific data flows. ESNet provides connectivity for all major DOE sites and facilities, and the network transports roughly 35 petabytes of traffic each month.
Berkeley Lab is the lead partner in the DOE’s Joint Bioenergy Institute (JBEI), located in Emeryville, California. Other partners are the DOE’s Sandia National Laboratory, the University of California (UC) campuses of Berkeley and Davis, the Carnegie Institution for Science , and the DOE’s Lawrence Livermore National Laboratory (LLNL). JBEI’s primary scientific mission is to advance the development of the next generation of biofuels – liquid fuels derived from the solar energy stored in plant biomass. JBEI is one of three new U.S. Department of Energy (DOE) Bioenergy Research Centers (BRCs).
Berkeley Lab has a major role in two DOE Energy Innovation Hubs. The mission of the Joint Center for Artificial Photosynthesis (JCAP) is to find a cost-effective method to produce fuels using only sunlight, water, and carbon dioxide. The lead institution for JCAP is the California Institute of Technology and Berkeley Lab is the second institutional center. The mission of the Joint Center for Energy Storage Research (JCESR) is to create next-generation battery technologies that will transform transportation and the electricity grid. The DOE’s Argonne National Laboratory leads JCESR and Berkeley Lab is a major partner.





