From The DOE’s Brookhaven National Laboratory
And
6.11.24
Karen McNulty Walsh
kmcnulty@bnl.gov
(631) 344-8350
Peter Genzer
genzer@bnl.gov
(631) 344-3174
Successful demonstration may boost production of hydrogen from water.
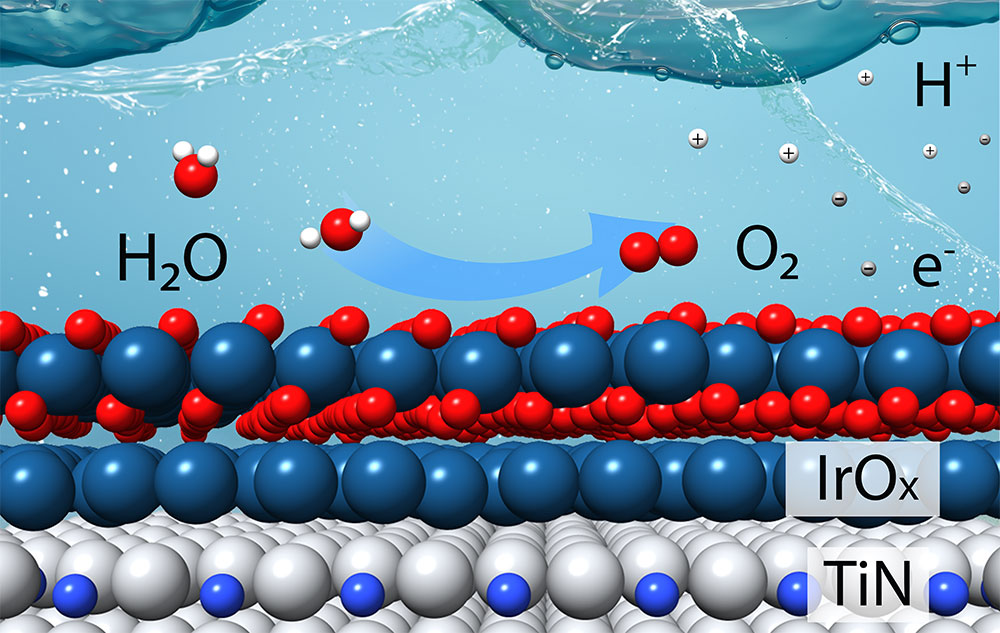
This schematic shows how a catalyst composed of a few layers of iridium oxide (IrOx) over a support made of titanium nitride (TiN) can efficiently produce oxygen (O2), hydrogen ions (H+), and electrons (e-) from water molecules (H2O) in an acidic electrolyte. This “oxygen evolution reaction” is the more challenging of two reactions needed to split water to produce hydrogen gas (H2). (Tianyou Mou/Brookhaven National Laboratory)
Hydrogen (H2) is a promising fuel for reducing greenhouse gases, especially if produced by using renewable energy to split water molecules (H2O). But as simple as it may seem to break water into hydrogen and oxygen, the chemistry is complex. Two separate simultaneous electrochemical reactions each require catalysts, chemical “deal makers” that help break and remake chemical bonds. Now, scientists at the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory and Columbia University say they’ve developed a new efficient catalyst for the more challenging part: the oxygen evolution reaction.
As described in a paper just published in the Journal of the American Chemical Society, the catalyst was designed “from the bottom up” based on theoretical calculations seeking to minimize the amount of iridium, an expensive metal used as a catalytic material, and to maximize the catalyst’s stability in acidic conditions. When the team created models of the catalyst and tested them in the lab, the results validated the predictions. Then, the scientists made a powder form of the catalyst, like those used in industrial applications, and showed it can efficiently produce hydrogen in a water-splitting electrolyzer.
“In this real-world test, our catalyst is about four times better than the state-of-the-art commercially available iridium catalyst,” said Jingguang Chen, a chemical engineer at Columbia University with a joint appointment in the Chemistry Division at Brookhaven Lab who led the research. In other words, the new catalyst requires four times less iridium to produce hydrogen at the same rate as the commercial variety — or produces hydrogen four times faster for the same amount of iridium.
Brookhaven Lab theoretical chemist Ping Liu, who led the calculations that underpin the catalyst’s design, said, “This study demonstrates how you can go from a theory-driven understanding of what’s happening at the atomic level to designing a catalyst for a practical use. Our work gives us a better understanding of how this catalyst works and gets us closer to the real-world application.”
The remaining challenge is to scale up production.
“We are only making milligrams of catalyst per batch,” Chen said. “If you want to make megatons of green hydrogen, you’d need kilograms or tons of catalyst. We can’t make this at that large scale yet.”
Reducing iridium
Iridium is the catalyst of choice for the oxygen evolution reaction, which takes place at the anode of an electrolyzer. It provides the electrically charged active sites that separate tightly bound hydrogen ions (H+) from oxygen (O). In addition to freeing the H+ ions — which contribute to the harshly acidic reaction conditions — the reaction produces oxygen gas (O2) and electrons. Those electrons are needed for the second, less challenging “hydrogen evolution” reaction: the pairing up of hydrogen ions to form hydrogen gas at the electrolyzer’s cathode.
“Iridium is currently one of the only stable elements for the oxygen evolution reaction in acid,” Chen said. That’s “unfortunate,” he noted, because “iridium is even more rare, and more expensive, than platinum.”
Hence, the motivation for reducing the amount of iridium.
“In industrial catalysts made of nanoscale particles, only atoms on the surface participate in the reaction,” Chen said. “That means most of the iridium on the inside of the particle is wasted.”
Maybe instead of using a particle that is all iridium, a catalyst could be made of a less-expensive material with iridium only on the surface, the team reasoned.
With funding from a DOE initiative to advance clean-energy technologies, they had been exploring the use of earth-abundant elements such as titanium. They found that combining titanium with nitrogen provided enough stability for these “titanium nitrides” to survive acidic reaction conditions. Perhaps titanium nitride could serve as the core of iridium-coated catalytic particles.
But how much iridium should be layered on top? This is where the theoretical calculations come in.
Calculating an ideal structure
“We used ‘density functional theory’ calculations to model how different overlayers of iridium on titanium nitride would affect the stability and activity of the catalyst under acidic oxygen evolution reaction conditions,” said Liu. She and her team used computing resources at Brookhaven Lab’s Center for Functional Nanomaterials (CFN) [below] and at the National Energy Research Scientific Computing Center (NERSC) at DOE’s Lawrence Berkeley National Laboratory to run the simulations.
The calculations predicted that one layer of iridium would not be sufficient to drive the oxygen evolution reaction but that two or three layers would improve both performance and catalytic stability.
Validating the predictions
First, the team created thin films in which they could create carefully controlled layers that closely resembled the surfaces used in the theoretical modeling calculations. They also created powdered samples composed of small nanoscale particles, the form the catalyst would take in industrial applications. Then, they studied the thin films — including the interfaces between the layers — and the nanoparticles using a variety of techniques.
These included transmission electron microscopy at CFN and X-ray spectroscopy studies at the Quick X-ray Absorption and Scattering (QAS) beamline of the National Synchrotron Light Source II (NSLS-II) [below], a source of bright X-rays for deciphering samples’ chemical and physical properties.
“Our hypothesis was that if the iridium bonds to the titanium nitride, this bonding would stabilize the iridium and improve the reaction,” Chen said.
The characterization studies bore out the predictions.
“The synchrotron studies revealed the oxidation states and local coordination environment of the iridium and titanium atoms under reaction conditions,” Chen said. “They confirmed that the iridium and titanium are interacting strongly.”
“Mapping the elements of the nanoparticles at CFN confirmed the particle sizes and compositions, including the presence of iridium oxides on the surface over titanium nitride supports,” he added.
Liu emphasized that the characterization studies informed the scientists’ understanding of the catalyst.
“We found that the interaction between iridium and titanium is not only helpful to the stability of the catalyst but also in fine tuning its activity,” she said. “The charges change the chemistry in a way that improves the reaction.”
Specifically, charges transferred from titanium to the iridium surface alter the electronic structure of the iridium active sites to optimize the binding of reaction intermediates, she explained.
“Going from one to three layers of iridium, you increase the charge transfer from the nitride to the top iridium significantly,” Liu noted. But the difference between two and three layers was not very large. Two layers might be enough to allow high stability, activity, and low cost.
To make this catalyst ready for real-world use, the scientists pointed out that, in addition to tackling the challenge of scaling up production, there could also be improvements to optimize consistency of the powders.
“When we make thin films, we can control the layers, but with powder synthesis, we don’t have that kind of control,” Chen said. “Our powder particles don’t have a continuous iridium shell around them. But this study provides guidelines industrial chemists could use to make true core-shell structures with a uniform thin layer of iridium,” he said.
Such catalysts could help lower the cost of water splitting and bring scientists closer to producing large quantities of green hydrogen.
This work was funded by the DOE Office of Science. CFN, NSLS-II, and NERSC all operate as DOE Office of Science user facilities.
“These were sort of pre-screening experiments,” Liu said. “Then, we turned these screening results over to the experimental team to make real catalysts and evaluate their catalytic activity.”
See the full article here .
Comments are invited and will be appreciated, especially if the reader finds any errors which I can correct.

five-ways-keep-your-child-safe-school-shootings
Please help promote STEM in your local schools.
![]()
Stem Education Coalition
Columbia University was founded in 1754 as King’s College by royal charter of King George II of England. It is the oldest institution of higher learning in the state of New York and the fifth oldest in the United States.
University Mission Statement
Columbia University is one of the world’s most important centers of research and at the same time a distinctive and distinguished learning environment for undergraduates and graduate students in many scholarly and professional fields. The University recognizes the importance of its location in New York City and seeks to link its research and teaching to the vast resources of a great metropolis. It seeks to attract a diverse and international faculty and student body, to support research and teaching on global issues, and to create academic relationships with many countries and regions. It expects all areas of the University to advance knowledge and learning at the highest level and to convey the products of its efforts to the world.
Columbia University is a private Ivy League research university in New York City. Established in 1754 on the grounds of Trinity Church in Manhattan Columbia is the oldest institution of higher education in New York and the fifth-oldest institution of higher learning in the United States. It is one of nine colonial colleges founded prior to the Declaration of Independence, seven of which belong to the Ivy League. Columbia is ranked among the top universities in the world by major education publications.
Columbia was established as King’s College by royal charter from King George II of Great Britain in reaction to the founding of Princeton College. It was renamed Columbia College in 1784 following the American Revolution, and in 1787 was placed under a private board of trustees headed by former students Alexander Hamilton and John Jay. In 1896, the campus was moved to its current location in Morningside Heights and renamed Columbia University.
Columbia scientists and scholars have played an important role in scientific breakthroughs including brain-computer interface; the laser and maser; nuclear magnetic resonance; the first nuclear pile; the first nuclear fission reaction in the Americas; the first evidence for plate tectonics and continental drift; and much of the initial research and planning for the Manhattan Project during World War II. Columbia is organized into twenty schools, including four undergraduate schools and 15 graduate schools. The university’s research efforts include the Lamont–Doherty Earth Observatory, the Goddard Institute for Space Studies, and accelerator laboratories with major technology firms such as IBM. Columbia is a founding member of the Association of American Universities and was the first school in the United States to grant the M.D. degree. With over 14 million volumes, Columbia University Library is the third largest private research library in the United States.
The university’s endowment stands among the largest of any academic institution. Columbia’s alumni, faculty, and staff have included: Founding Fathers of the United States—among them a co-author of the United States Constitution and a co-author of the Declaration of Independence; U.S. presidents; foreign heads of state; justices of the United States Supreme Court; Nobel laureates; Fields Medalists; many members of National Academy of Sciences; living billionaires; Olympic medalists; Academy Award winners; and Pulitzer Prize recipients.

One of ten national laboratories overseen and primarily funded by the The DOE Office of Science, The DOE’s Brookhaven National Laboratory conducts research in the physical, biomedical, and environmental sciences, as well as in energy technologies and national security. Brookhaven Lab also builds and operates major scientific facilities available to university, industry and government researchers. The Laboratory’s almost 3,000 scientists, engineers, and support staff are joined each year by more than 5,000 visiting researchers from around the world. Brookhaven is operated and managed for DOE’s Office of Science by Brookhaven Science Associates, a limited-liability company founded by Stony Brook University the largest academic user of Laboratory facilities, and Battelle, a nonprofit, applied science and technology organization.
Research at BNL specializes in nuclear and high energy physics, energy science and technology, environmental and bioscience, nanoscience and national security. The 5300 acre campus contains several large research facilities, including the Relativistic Heavy Ion Collider [below] and National Synchrotron Light Source II [below]. A number of Nobel prizes have been awarded for work conducted at Brookhaven lab.
BNL is staffed by approximately 2,750 scientists, engineers, technicians, and support personnel, and hosts 4,000 guest investigators every year. The laboratory has its own police station, fire department, and ZIP code (11973). In total, the lab spans a 5,265-acre (21 km^2) area that is mostly coterminous with the hamlet of Upton, New York. BNL is served by a rail spur operated as-needed by the New York and Atlantic Railway. Co-located with the laboratory is the Upton, New York, forecast office of the National Weather Service.
Major programs
Although originally conceived as a nuclear research facility, Brookhaven Lab’s mission has greatly expanded. Its foci are now:
Nuclear and high-energy physics
Physics and chemistry of materials
Environmental and climate research
Nanomaterials
Energy research
Nonproliferation
Structural biology
Accelerator physics
Operation
Brookhaven National Lab was originally owned by the Atomic Energy Commission and is now owned by that agency’s successor, the United States Department of Energy (DOE). DOE subcontracts the research and operation to universities and research organizations. It is currently operated by Brookhaven Science Associates LLC, which is an equal partnership of Stony Brook University and Battelle Memorial Institute. From 1947 to 1998, it was operated by Associated Universities, Inc. (AUI), but AUI lost its contract in the wake of two incidents: a 1994 fire at the facility’s high-beam flux reactor that exposed several workers to radiation and reports in 1997 of a tritium leak into the groundwater of the Long Island Central Pine Barrens on which the facility sits.
Foundations
Following World War II, the US Atomic Energy Commission was created to support government-sponsored peacetime research on atomic energy. The effort to build a nuclear reactor in the American northeast was fostered largely by physicists Isidor Isaac Rabi and Norman Foster Ramsey Jr., who during the war witnessed many of their colleagues at Columbia University leave for new remote research sites following the departure of the Manhattan Project from its campus. Their effort to house this reactor near New York City was rivalled by a similar effort at the Massachusetts Institute of Technology to have a facility near Boston, Massachusetts. Involvement was quickly solicited from representatives of northeastern universities to the south and west of New York City such that this city would be at their geographic center. In March 1946 a nonprofit corporation was established that consisted of representatives from nine major research universities — Columbia University, Cornell University, Harvard University, Johns Hopkins University, Massachusetts Institute of Technology, Princeton University, University of Pennsylvania, University of Rochester, and Yale University.
Out of 17 considered sites in the Boston-Washington corridor, Camp Upton on Long Island was eventually chosen as the most suitable in consideration of space, transportation, and availability. The camp had been a training center from the US Army during both World War I and World War II. After the latter war, Camp Upton was deemed no longer necessary and became available for reuse. A plan was conceived to convert the military camp into a research facility.
On March 21, 1947, the Camp Upton site was officially transferred from the U.S. War Department to the new U.S. Atomic Energy Commission (AEC), predecessor to the U.S. Department of Energy (DOE).
Research and facilities
Reactor history
In 1947 construction began on the first nuclear reactor at Brookhaven, the Brookhaven Graphite Research Reactor. This reactor, which opened in 1950, was the first reactor to be constructed in the United States after World War II. The High Flux Beam Reactor operated from 1965 to 1999. In 1959 Brookhaven built the first US reactor specifically tailored to medical research, the Brookhaven Medical Research Reactor, which operated until 2000.
Accelerator history
In 1952 Brookhaven began using its first particle accelerator, the Cosmotron. At the time the Cosmotron was the world’s highest energy accelerator, being the first to impart more than 1 GeV of energy to a particle.

The Cosmotron was retired in 1966, after it was superseded in 1960 by the new Alternating Gradient Synchrotron (AGS).
 BNL Alternating Gradient Synchrotron (AGS).
BNL Alternating Gradient Synchrotron (AGS).
The AGS was used in research that resulted in 3 Nobel prizes, including the discovery of the muon neutrino, the charm quark, and CP violation.
In 1970 in BNL started the ISABELLE project to develop and build two proton intersecting storage rings.
The groundbreaking for the project was in October 1978. In 1981, with the tunnel for the accelerator already excavated, problems with the superconducting magnets needed for the ISABELLE accelerator brought the project to a halt, and the project was eventually cancelled in 1983.

After ISABELLE’S cancellation, physicist at BNL proposed that the excavated tunnel and parts of the magnet assembly be used in another accelerator. In 1984 the first proposal for the accelerator now known as the Relativistic Heavy Ion Collider (RHIC)[below] was put forward. The construction got funded in 1991 and RHIC has been operational since 2000. One of the world’s only two operating heavy-ion colliders, RHIC is as of 2010 the second-highest-energy collider after the Large Hadron Collider (CH). RHIC is housed in a tunnel 2.4 miles (3.9 km) long and is visible from space.
On January 9, 2020, it was announced by Paul Dabbar, undersecretary of the US Department of Energy Office of Science, that the BNL eRHIC design has been selected over the conceptual design put forward by DOE’s Thomas Jefferson National Accelerator Facility [Jlab] as the future Electron–ion collider (EIC)

In addition to the site selection, it was announced that the BNL EIC had acquired CD-0 from the Department of Energy. BNL’s eRHIC design proposes upgrading the existing Relativistic Heavy Ion Collider, which collides beams light to heavy ions including polarized protons, with a polarized electron facility, to be housed in the same tunnel.
Other discoveries
In 1958, Brookhaven scientists created one of the world’s first video games, Tennis for Two. In 1968 Brookhaven scientists patented Maglev, a transportation technology that utilizes magnetic levitation.
Major facilities
Relativistic Heavy Ion Collider (RHIC), which was designed to research quark–gluon plasma and the sources of proton spin. Until 2009 it was the world’s most powerful heavy ion collider. It is the only collider of spin-polarized protons.
Center for Functional Nanomaterials (CFN), used for the study of nanoscale materials.
BNL National Synchrotron Light Source II, Brookhaven’s newest user facility, opened in 2015 to replace the National Synchrotron Light Source (NSLS), which had operated for 30 years. NSLS was involved in the work that won the 2003 and 2009 Nobel Prize in Chemistry.
Alternating Gradient Synchrotron, a particle accelerator that was used in three of the lab’s Nobel prizes.
Accelerator Test Facility, generates, accelerates and monitors particle beams.
Tandem Van de Graaff, once the world’s largest electrostatic accelerator.
Computational Science resources, including access to a massively parallel Blue Gene series supercomputer that is among the fastest in the world for scientific research, run jointly by Brookhaven National Laboratory and Stony Brook University-SUNY.
Interdisciplinary Science Building, with unique laboratories for studying high-temperature superconductors and other materials important for addressing energy challenges.
NASA Space Radiation Laboratory, where scientists use beams of ions to simulate cosmic rays and assess the risks of space radiation to human space travelers and equipment.
Off-site contributions
It is a contributing partner to the ATLAS experiment, one of the four detectors located at the The European Organization for Nuclear Research [La Organización Europea para la Investigación Nuclear][Organization européenne pour la recherche nucléaire] [Europäische Organization für Kernforschung](CH)[CERN] Large Hadron Collider(LHC). Credit: CERN.


It is currently operating at The European Organization for Nuclear Research [La Organización Europea para la Investigación Nuclear][Organization européenne pour la recherche nucléaire] [Europäische Organization für Kernforschung](CH) [CERN] near Geneva, Switzerland.
Brookhaven was also responsible for the design of Spallation Neutron Source at the DOE’s Oak Ridge National Laboratory, Tennessee.

Brookhaven plays a role in a range of neutrino research projects around the world, including the Daya Bay Neutrino Experiment (CN) nuclear power plant, approximately 52 kilometers northeast of Hong Kong and 45 kilometers east of Shenzhen, China.

















