From The DOE’s Pacific Northwest National Laboratory
6.12.24
Karyn Hede
Cooks love stainless steel for its durability, rust resistance and even cooking when heated. But few know the secret that makes stainless steel so popular. It’s the metal chromium in stainless steel, which reacts with oxygen in the air to form a stable and protective thin coating for protecting the steel underneath.
These days, scientists and engineers are working to design alloys that can resist extreme environments for applications such as nuclear fusion reactors, hypersonic flights and high-temperature jet engines. For such extreme applications, scientists are experimenting with complex combinations of many metals mixed in equal proportions in what are called multi-principal element alloys or medium- to high-entropy alloys. These alloys aim to achieve design goals such as strength, toughness, resistance to corrosion and so on. Specifically, researchers seek alloys resistant to corrosion that can happen when metals react with oxygen in the atmosphere, a process called oxidation. These alloys are typically tested in a “cook-and-look” procedure where alloy materials are exposed to high-temperature oxidation environments to see how they respond.
But now, a multidisciplinary research team led by scientists at the Department of Energy’s Pacific Northwest National Laboratory and North Carolina State University combined atomic-scale experiments with theory to create a tool to predict how such high-entropy alloys will behave under high-temperature oxidative environments. The research, published in the journal Nature Communications, offers a road map toward rapid design and testing cycles for oxidation-resistant complex metal alloys.
Fig. 1: Progression in speciation during oxidation of five component HEA.
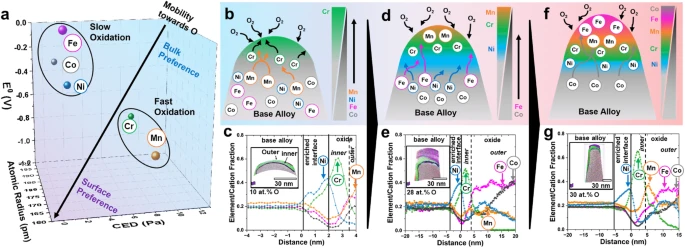
a Preferential interactivity parameter (PIP) for the elements involved in the HEA for early prediction of oxidation behavior. b Schematic of the elemental distribution during initial oxidation at t = 0. c Atom probe proximity histograms (proxigrams) of HEA needle’s top after oxidation at 400 °C for t = 2 min. d Schematic of the elemental distribution during initial oxidation at 0 < t < ∞. e Atom probe proxigrams of HEA after oxidation at 400 °C for t = 30 min. f Schematic of the elemental distribution during initial oxidation at t ~ ∞. g Atom probe proxigrams of HEA after oxidation at 400 °C for t = 120 min. Further details on the APT experimental results can be seen in the following section.
Fig. 2: Ex situ analysis of oxidized HEA (CoCrFeNiMn).
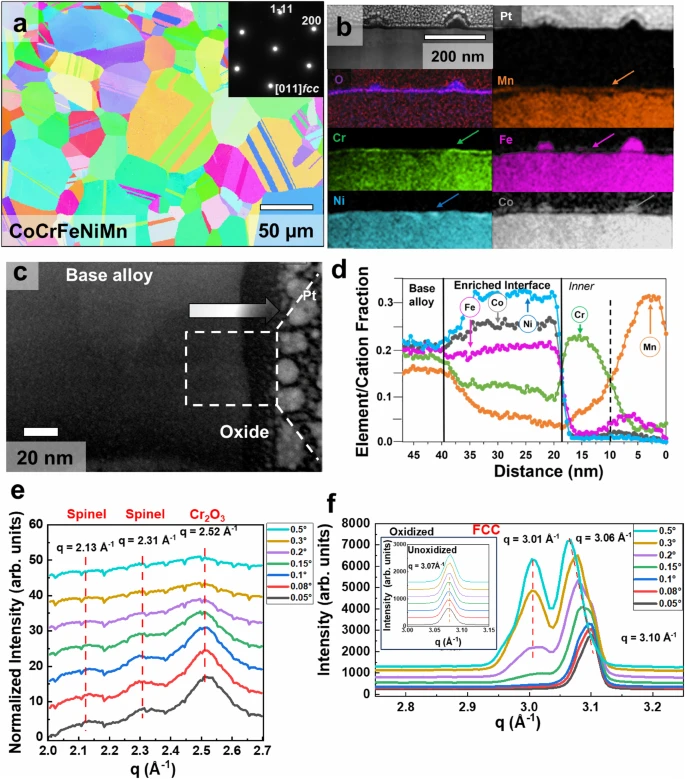
a Inverse pole figure map showing the randomly oriented recrystallized microstructure of the starting alloy condition. The inset shows the selected area diffraction pattern, further confirming the FCC structure and single phase. b A cross-section of the sample extracted using FIB after oxidation at 400 °C for 120 min at 10 mbar pressure inside the custom-made atom probe reactor chamber. The figure shows the STEM image and the elemental distribution in the oxide film and the base metal. c STEM image further magnifying the oxide film where an arrow is used to traverse from the base alloys to the oxide film. d TEM EDS linear profile of elemental distribution on traversing from base metal to oxide film. e GIWAXS traces from q = 2–2.7 Å^−1 and f from 2.75–3.25 Å^−1 with incidence angle varying from 0.05o (lower) to 0.5o (top). The lowest angle grazes the topmost oxide layer showing the presence of a Spinel structure and Corundum structure and as the grazing angle increases these phases disappear. Peak shift and peak splitting can be seen in (f) due to compositional changes in the matrix near the oxide metal interface.
See the science paper for further instructive material with images.
“We are working toward developing an atomic-scale model for material degradation of these complex alloys, which then can be applied to design next-generation alloys with superior resistance to extreme environments for a wide variety of applications such as the aerospace and nuclear power industries,” said Arun Devaraj, co-principal investigator of the study and a PNNL materials scientist specializing in understanding metal degradation in extreme environments. “The goal here is to find ways to rapidly identify medium- to high-entropy alloys with the desired properties and oxidation resistance for your chosen application.”

Materials scientist Arun Devaraj works at an atom probe tomography instrument at Pacific Northwest National Laboratory. This precision instrument can show the placement of atoms in tiny samples of materials, such as metal alloys. (Photo by Andrea Starr | Pacific Northwest National Laboratory)
A complex alloy recipe
For their recent experiments, the research team studied the degradation of a high-entropy alloy with equal amounts of the metals cobalt, chromium, iron, nickel and manganese (CoCrFeNiMn, also called the Cantor alloy). The research team examined oxide formed on the Cantor alloy using a variety of advanced atomic-scale methods to understand how each element arranges itself in the alloy and the oxide.
They discovered that chromium and manganese tend to migrate quickly toward the surface and form stable chromium and manganese oxides. Subsequently, iron and cobalt diffuse through these oxides to form additional layers.
By adding a small amount of aluminum, they discovered that aluminum oxide can act as a barrier for other elements migrating to form the oxide, thereby reducing the overall oxidation of the aluminum-containing Cantor alloy and increasing its resistance to degradation at high temperatures.

Microscopic samples of complex metal alloy are placed on a sampling vessel to enter the atom probe tomography instrument. (Photo by Andrea Starr | Pacific Northwest National Laboratory)
“This work sheds light on the mechanisms of oxidation in complex alloys at the atomic scale,” said Bharat Gwalani, co-corresponding author of the study. Gwalani began the study while a scientist at PNNL and continued the research in his current role as an assistant professor of materials science and engineering at North Carolina State University. He added, “by understanding the fundamental mechanisms involved, this work gives us a deeper understanding of oxidation across all complex alloys.”
Predictive models
“Right now there are no universally applicable governing models to extrapolate how a given complex, multi-principal element alloy will oxidize and degrade over time in a high-temperature oxidation environment,” said Devaraj. “This is a substantial step in that direction.”
The team’s careful analysis revealed some universal rules that can predict how the oxidation process will proceed in these complex alloys. Computational colleagues from NCSU developed a model called the Preferential Interactivity Parameter for early prediction of oxidation behavior in complex metal alloys.
Ultimately, the research team expects to expand this research to develop complex alloys with exceptional high-temperature properties, and to do so very quickly by rapid sampling and analysis. The ultimate goal is to choose a combination of elements that favor the formation of an adherent oxide, said Devaraj. “You know oxide formation will happen, but you want to have a very stable oxide that will be protective, that would not change over time, and would withstand extreme heat inside a rocket engine or nuclear reactors.”
A next step will be to introduce automated experimentation and integrate additive manufacturing methods, along with advanced artificial intelligence, to rapidly evaluate promising new alloys. That project is now getting underway at PNNL as a part of the Adaptive Tunability for Synthesis and Control via the Autonomous Learning on Edge (AT SCALE) Initiative.
“That kind of discovery loop for materials discovery will be very relevant for further expanding our knowledge of these novel alloys,” said Devaraj, who also has a joint faculty appointment at the Colorado School of Mines.
In addition to Gwalani and Devaraj, PNNL scientists Sten Lambeets, Matthew Olszta, Anil Krishna Battu and Thevuthasan Suntharampillai contributed; as well as Martin Thuo, Aram Amassian, Andrew Martin, Aniruddha Malakar, and Boyu Guo of NCSU; Elizabeth Kautz, an assistant professor of nuclear engineering at North Carolina State, who also has a joint appointment with PNNL; Feipeng Yang and Jinghua Guo of the DOE’s Lawrence Berkeley National Laboratory; and Ruipeng Li of the DOE’s Brookhaven National Laboratory.
To investigate the arrangement of atoms within the samples, the research team used in situ atom probe tomography at PNNL. These results were correlated with electron microscopy and synchrotron-based grazing incidence wide-angle X-ray scattering at the National Synchrotron Light Source II, BNL, and X-ray absorption measurements conducted at the Advanced Light Source, LBNL, national user facilities funded by the DOE Office of Science.


The research was also supported in part by the DOE Office of Science, Basic Energy Sciences, Materials Sciences and Engineering Division as a part of the Early Career Research Program.
See the full article here.
Comments are invited and will be appreciated, especially if the reader finds any errors which I can correct.
five-ways-keep-your-child-safe-school-shootings
Please help promote STEM in your local schools.
The DOE’s Pacific Northwest National Laboratory (PNNL) is one of the United States Department of Energy National Laboratories, managed by the Department of Energy’s Office of Science. The main campus of the laboratory is in Richland, Washington.
PNNL scientists conduct basic and applied research and development to strengthen U.S. scientific foundations for fundamental research and innovation; prevent and counter acts of terrorism through applied research in information analysis, cyber security, and the nonproliferation of weapons of mass destruction; increase the U.S. energy capacity and reduce dependence on imported oil; and reduce the effects of human activity on the environment.
Facilities
PNNL houses several scientific user facilities and research facilities.
Scientific user facilities
The Environmental Molecular Sciences Laboratory (EMSL) is a U.S. Department of Energy national scientific user facility. EMSL provides researchers around the world with integrated capabilities in oxide and mineral interface chemistry, high-performance computing and computational chemistry software, mass spectrometry, high-field magnetic resonance, and subsurface flow and transport.
The Bioproducts, Sciences, and Engineering Laboratory (BSEL) is a joint effort between The Washington State University and PNNL, and is located on the WSU-Tri-Cities campus. Within BSEL, researchers are developing technology for converting agricultural byproducts into chemicals for products like plastics, solvents, fibers, pharmaceuticals, and fuel additives.
Researchers at PNNL’s Radiochemical Processing Laboratory are developing processes to advance the cleanup of radiological and hazardous wastes; the processing and disposal of nuclear fuels; and the production and delivery of medical isotopes.
The Applied Process Engineering Laboratory (APEL) is a technology business startup user facility, sponsored in part by PNNL. APEL provides engineering- and manufacturing-scale space and chemical, biological, and electronic laboratories and equipment for developing, validating, and commercializing new products.
Research facilities
Three research facilities were constructed on PNNL’s Richland, Washington campus to partially replace laboratory and office space PNNL had been using on the south end of the nearby Hanford Site.
The Physical Sciences Facility, a federally funded research complex that was designed by Flad Architects, opened in 2010 houses PNNL’s research into materials science, radiation detection, and ultra-trace analysis. The privately funded Computational Sciences Facility and Biological Sciences Facility house about 310 staff who support PNNL’s energy, environmental, national security, and fundamental science research missions. These two new facilities opened in 2009. The CSF contains scientific capabilities in information analytics, high-performance computing, cyber security and bioinformatics. The BSF focuses on bioenergy, environmental and soil remediation and includes systems biology, microbial and cellular biology and analytical interfacial chemistry.
The Electricity Infrastructure Operations Center at PNNL combines software, real-time power grid data and computation into a control room setting. The ideas and technologies developed in the EIOC address better management of the power grid. The EIOC also is available to utilities, vendors, government agencies and universities interested in research, development or training.
PNNL-Sequim (2022–present), previously known as the Marine and Coastal Research Laboratory (2021) and the Marine Sciences Laboratory (1966–2021), located at Sequim, Washington, is the DOE’s only marine laboratory. PNNL-Sequim provides analytical and general-purpose laboratories, as well as wet or support laboratories supplied with heated and cooled freshwater and seawater. More than 20 engineers and scientists work on coastal restoration and security projects, from reviving salmon habitat to research on how shellfish could detect a bioterrorist attack. PNNL-Sequim also operates a 28-foot (8.5 m) research vessel.
Other PNNL research facilities include the following:
Research Aircraft
Pretreatment Engineering Platform
Microproducts Breakthrough Institute
Joint Global Change Research Institute
Instrument Performance Testing
Hanford Meteorological Station
In Vivo Radioassay and Research Facility
Non-Destructive Analysis Laboratory
Radiological Calibration and Irradiation Facility
Proteomics other Mass Spectrometry-based Omics
Shallow underground laboratory for low-activity radiation measurement
The Laboratory has been operated by Ohio-based Battelle since 1965





