From New Jersey Institute of Technology
via
Exploring how the multiscale interaction between underwater oil and gas plumes and the environment impacts plume composition and trajectory.
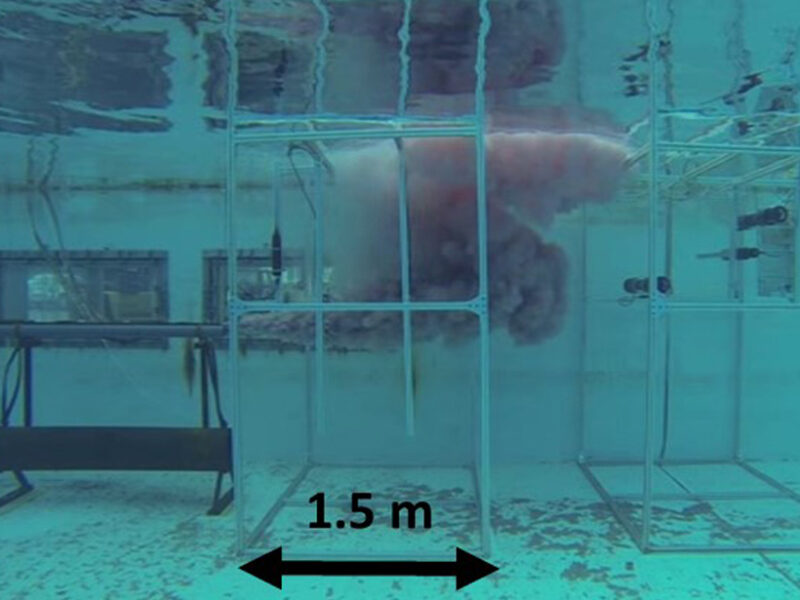
Hydrocarbon plumes released to the environment manifest spontaneous changes through the formation of oil droplets and gas bubbles and the release of soluble compounds in the water column. This tank experiment is observing a flow of diesel oil blended with fluorescein dye escaping from a one-inch (2.5 centimeter) pipe at the flow of 500 liters per minute. Major advances have been made for understanding the oil/gas chemistry and physics at depth and the behavior of multiphase plumes (oil, gas, and water). Credit: study conducted by New Jersey Institute of Technology and collaborators at the Department of Interior Ohmsett facility in New Jersey.
9.1.20
Michel C. Boufadel
Scott A. Socolofsky
Natural seeps on the continental margins release hydrocarbons in the form of liquid oil and natural gas. Hydrocarbons may also be leaked from sunken vessels and damaged pipelines. The most dramatic example of an anthropogenic hydrocarbon release in recent history is the Deepwater Horizon oil well blowout in the Northern Gulf of Mexico in 2010. A recent article in Reviews of Geophysics explores how hydrocarbon liquids and gases behave after being released underwater. Here, two of the authors give an overview of how oil droplets and gas bubbles move around in aquatic environments.
How do the characteristics of hydrocarbon liquids and gases change after contact with water?
The majority of chemical compounds in oil and gas do not dissolve in water so, once in contact with seawater they do not mix but rather break up into droplets and bubbles.

Sequence of photos showing crude oil during release from a 1.0-centimeter pipe to evaluate the oil droplet size distribution. Credit: Study conducted by New Jersey Institute of Technology and collaborators at the Department of Interior Ohmsett facility in New Jersey.
The sizes of droplets and bubbles depends on the balance between the destructive force of mixing energy in various compartments of the environment, particularly the release point (vent, pipe, etc..) and the resistive force mainly due to the interfacial tension between the oil and water or gas and water (the interfacial tension force is the physical factor behind the statement “oil and water do no mix”).
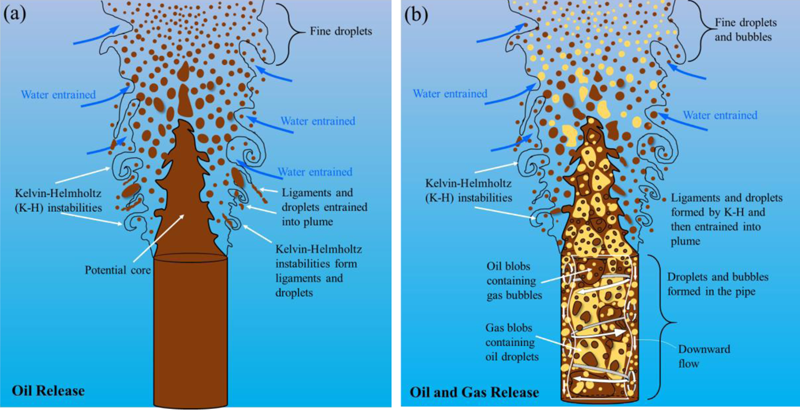
The flow of gas impacts the plume and the size of the oil droplets (a) In oil-only releases, ligaments form at the interface of oil and water and they get entrained into plume and subsequently disintegrate due to turbulence within the plume. An intact core of oil exists within a few diameters of the orifice, (b) For oil and gas release, oil droplets form also in the center of the jet, especially if the flow is “churn” (where oil and gas tumble within the conduit). Credit: Boufadel et al. [2020], Figure 6.
In deep seawater, where there are conditions of high pressure and low temperature, the release of hydrocarbon compounds can form natural gas hydrates. These are solid deposits with an ice-like crystalline structure that changes the solubility and bioavailability of the hydrated compounds.
How do they behave as they rise through the water column?
A large volume of hydrocarbon forms an underwater plume. As the plume rises, it entrains water in it, and eventually each individual oil droplet and gas bubble continues rising at its “terminal” velocity, which results from a balance between its buoyancy and its drag.
During ascent, small droplets (less than 300 microns) drift with the currents, while larger ones tend to rise vertically to the surface. Droplets and bubbles lose mass as they rise due to dissolution in water.
How far and fast can they travel in the aquatic environment?
Gas bubbles typically rise at velocities between 10 and 30 centimeters per second, with greater speeds associated with large bubbles. Large (millimeter-scale) oil droplets rise at similar speeds of up to 20 centimeters per second; smaller oil droplets can rise very slowly while micro-scale oil droplets have hardly move upwards at all.
The lateral transport of oil droplets and gas bubbles depend on two main factors: currents and the rise time from depth. Gas bubbles surface in minutes to hours, limiting the lateral drift to a scale of kilometers. Large oil droplets have similar rise times and lateral movements. In contrast, small oil droplets can remain sequestered in the ocean water column for long periods and be transported tens to hundreds of kilometers from the release point.
In the case of an oil-well blowout, the collective buoyancy of the oil and gas can form a plume, which may rise at up to 1 meter per second, carrying a whole collection of gas bubbles and oil droplets collectively upward from the source. In the case of the Deepwater Horizon, the plume of oil and gas was trapped by the ocean density stratification. This created a lateral intrusion of dissolved gas, light hydrocarbons, and very small oil droplets. Larger oil droplets rose out of the intrusion layer and surfaced largely within kilometers of the blowout footprint on the water surface.
What have been some recent advances in our understanding?
There has been tremendous improvement in models to describe oil droplet and gas bubble formation especially at depth, as well as observations in the laboratory at the submillimeter scale to validate them. There have also been advances in “fate models” to understand the real-fluid chemistry and thermodynamics of oil and gas in the ocean water column.
Meanwhile, new computational fluid dynamics (CFD) models for jet and plume dynamics of oil and gas in seawater have seen transformative advances.
What are some of the unresolved questions where additional research, data or modeling is needed?
Despite numerous laboratory and field observations of oil and gas behavior in seawater, we still lack observations of complete oil droplet size distribution or complete gas bubble size distribution from large orifices (10 centimeters or larger) under highly turbulent conditions. This is further complicated by the behavior of gas under high pressure (gas density is 1 kilogram per cubic meter at the water surface but could reach 150 kilograms per cubic meter at depth).
As of yet, computational fluid dynamic models are unable to capture the droplet or bubble fragmentation process, which is the result of forces in various fluids interacting at the micron scale (with different equations for different fluids). In addition, the effectiveness of added dispersants is not fully quantified in the presence of three phases: water, oil, and gas.
See the full article here .
five-ways-keep-your-child-safe-school-shootings
Please help promote STEM in your local schools.
Eos is the leading source for trustworthy news and perspectives about the Earth and space sciences and their impact. Its namesake is Eos, the Greek goddess of the dawn, who represents the light shed on understanding our planet and its environment in space by the Earth and space sciences.
Welcome to the New Jersey Institute of Technology. We’re proud of our 130 years of history, but that’s only the beginning of our story – we’ve doubled the size of our campus in the last decade, pouring millions into major new research facilities to give our students the edge they need in today’s demanding high-tech marketplace.
NJIT offers 125 undergraduate and graduate degree programs in six specialized schools instructed by expert faculty, 98 percent of whom hold the highest degree in their field.
Our academic programs are fully accredited by the appropriate accrediting boards, commissions and associations such as Middle States, ABET, and NAAB.



