January 19, 2017
Natalie Wolchover
Researchers have discovered that simple “chemically active” droplets grow to the size of cells and spontaneously divide, suggesting they might have evolved into the first living cells.

davidope for Quanta Magazine
A collaboration of physicists and biologists in Germany has found a simple mechanism that might have enabled liquid droplets to evolve into living cells in early Earth’s primordial soup.
Origin-of-life researchers have praised the minimalism of the idea. Ramin Golestanian, a professor of theoretical physics at the University of Oxford who was not involved in the research, called it a big achievement that suggests that “the general phenomenology of life formation is a lot easier than one might think.”
The central question about the origin of life has been how the first cells arose from primitive precursors. What were those precursors, dubbed “protocells,” and how did they come alive? Proponents of the “membrane-first” hypothesis have argued that a fatty-acid membrane was needed to corral the chemicals of life and incubate biological complexity. But how could something as complex as a membrane start to self-replicate and proliferate, allowing evolution to act on it?
In 1924, Alexander Oparin, the Russian biochemist who first envisioned a hot, briny primordial soup as the source of life’s humble beginnings, proposed that the mystery protocells might have been liquid droplets — naturally forming, membrane-free containers that concentrate chemicals and thereby foster reactions. In recent years, droplets have been found to perform a range of essential functions inside modern cells, reviving Oparin’s long-forgotten speculation about their role in evolutionary history. But neither he nor anyone else could explain how droplets might have proliferated, growing and dividing and, in the process, evolving into the first cells.
Now, the new work by David Zwicker and collaborators at the Max Planck Institute for the Physics of Complex Systems and the Max Planck Institute of Molecular Cell Biology and Genetics, both in Dresden, suggests an answer. The scientists studied the physics of “chemically active” droplets, which cycle chemicals in and out of the surrounding fluid, and discovered that these droplets tend to grow to cell size and divide, just like cells. This “active droplet” behavior differs from the passive and more familiar tendencies of oil droplets in water, which glom together into bigger and bigger droplets without ever dividing.
If chemically active droplets can grow to a set size and divide of their own accord, then “it makes it more plausible that there could have been spontaneous emergence of life from nonliving soup,” said Frank Jülicher, a biophysicist in Dresden and a co-author of the new paper.
The findings, reported in Nature Physics last month, paint a possible picture of life’s start by explaining “how cells made daughters,” said Zwicker, who is now a postdoctoral researcher at Harvard University. “This is, of course, key if you want to think about evolution.”
Luca Giomi, a theoretical biophysicist at Leiden University in the Netherlands who studies the possible physical mechanisms behind the origin of life, said the new proposal is significantly simpler than other mechanisms of protocell division that have been considered, calling it “a very promising direction.”
However, David Deamer, a biochemist at the University of California, Santa Cruz, and a longtime champion of the membrane-first hypothesis, argues that while the newfound mechanism of droplet division is interesting, its relevance to the origin of life remains to be seen. The mechanism is a far cry, he noted, from the complicated, multistep process by which modern cells divide.
Could simple dividing droplets have evolved into the teeming menagerie of modern life, from amoebas to zebras? Physicists and biologists familiar with the new work say it’s plausible. As a next step, experiments are under way in Dresden to try to observe the growth and division of active droplets made of synthetic polymers that are modeled after the droplets found in living cells. After that, the scientists hope to observe biological droplets dividing in the same way.
Clifford Brangwynne, a biophysicist at Princeton University who was part of the Dresden-based team that identified the first subcellular droplets eight years ago — tiny liquid aggregates of protein and RNA in cells of the worm C. elegans — explained that it would not be surprising if these were vestiges of evolutionary history. Just as mitochondria, organelles that have their own DNA, came from ancient bacteria that infected cells and developed a symbiotic relationship with them, “the condensed liquid phases that we see in living cells might reflect, in a similar sense, a sort of fossil record of the physicochemical driving forces that helped set up cells in the first place,” he said.
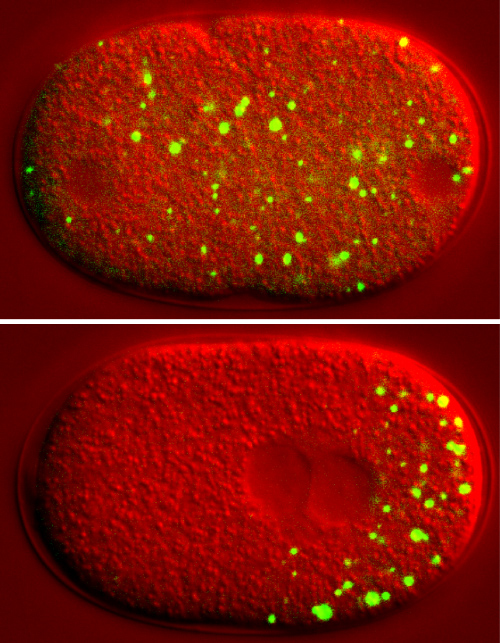
When germline cells in the roundworm C. elegans divide, P granules, shown in green, condense in the daughter cell that will become a viable sperm or egg and dissolve in the other daughter cell. Courtesy of Clifford Brangwynne/Science
“This Nature Physics paper takes that to the next level,” by revealing the features that droplets would have needed “to play a role as protocells,” Brangwynne added.
Droplets in Dresden
The Dresden droplet discoveries began in 2009, when Brangwynne and collaborators demystified the nature of little dots known as “P granules” in C. elegans germline cells, which undergo division into sperm and egg cells. During this division process, the researchers observed that P granules grow, shrink and move across the cells via diffusion. The discovery that they are liquid droplets, reported in Science, prompted a wave of activity as other subcellular structures were also identified as droplets. It didn’t take long for Brangwynne and Tony Hyman, head of the Dresden biology lab where the initial experiments took place, to make the connection to Oparin’s 1924 protocell theory. In a 2012 essay about Oparin’s life and seminal book, The Origin of Life, Brangwynne and Hyman wrote that the droplets he theorized about “may still be alive and well, safe within our cells, like flies in life’s evolving amber.”
Oparin most famously hypothesized that lightning strikes or geothermal activity on early Earth could have triggered the synthesis of organic macromolecules necessary for life — a conjecture later made independently by the British scientist John Haldane and triumphantly confirmed by the Miller-Urey experiment in the 1950s. Another of Oparin’s ideas, that liquid aggregates of these macromolecules might have served as protocells, was less celebrated, in part because he had no clue as to how the droplets might have reproduced, thereby enabling evolution. The Dresden group studying P granules didn’t know either.
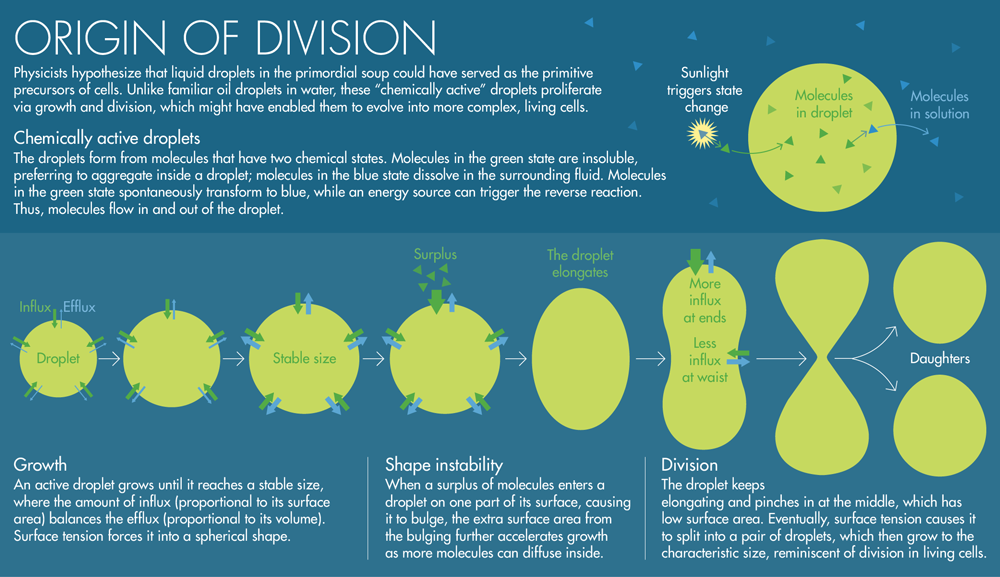
In the wake of their discovery, Jülicher assigned his new student, Zwicker, the task of unraveling the physics of centrosomes, organelles involved in animal cell division that also seemed to behave like droplets. Zwicker modeled the centrosomes as “out-of-equilibrium” systems that are chemically active, continuously cycling constituent proteins into and out of the surrounding liquid cytoplasm. In his model, these proteins have two chemical states. Proteins in state A dissolve in the surrounding liquid, while those in state B are insoluble, aggregating inside a droplet. Sometimes, proteins in state B spontaneously switch to state A and flow out of the droplet. An energy source can trigger the reverse reaction, causing a protein in state A to overcome a chemical barrier and transform into state B; when this insoluble protein bumps into a droplet, it slinks easily inside, like a raindrop in a puddle. Thus, as long as there’s an energy source, molecules flow in and out of an active droplet. “In the context of early Earth, sunlight would be the driving force,” Jülicher said.
Zwicker discovered that this chemical influx and efflux will exactly counterbalance each other when an active droplet reaches a certain volume, causing the droplet to stop growing. Typical droplets in Zwicker’s simulations grew to tens or hundreds of microns across depending on their properties — the scale of cells.
Lucy Reading-Ikkanda/Quanta Magazine
The next discovery was even more unexpected. Although active droplets have a stable size, Zwicker found that they are unstable with respect to shape: When a surplus of B molecules enters a droplet on one part of its surface, causing it to bulge slightly in that direction, the extra surface area from the bulging further accelerates the droplet’s growth as more molecules can diffuse inside. The droplet elongates further and pinches in at the middle, which has low surface area. Eventually, it splits into a pair of droplets, which then grow to the characteristic size. When Jülicher saw simulations of Zwicker’s equations, “he immediately jumped on it and said, ‘That looks very much like division,’” Zwicker said. “And then this whole protocell idea emerged quickly.”
Zwicker, Jülicher and their collaborators, Rabea Seyboldt, Christoph Weber and Tony Hyman, developed their theory over the next three years, extending Oparin’s vision. “If you just think about droplets like Oparin did, then it’s not clear how evolution could act on these droplets,” Zwicker said. “For evolution, you have to make copies of yourself with slight modifications, and then natural selection decides how things get more complex.”
Globule Ancestor
Last spring, Jülicher began meeting with Dora Tang, head of a biology lab at the Max Planck Institute of Molecular Cell Biology and Genetics, to discuss plans to try to observe active-droplet division in action.
Tang’s lab synthesizes artificial cells made of polymers, lipids and proteins that resemble biochemical molecules. Over the next few months, she and her team will look for division of liquid droplets made of polymers that are physically similar to the proteins in P granules and centrosomes. The next step, which will be made in collaboration with Hyman’s lab, is to try to observe centrosomes or other biological droplets dividing, and to determine if they utilize the mechanism identified in the paper by Zwicker and colleagues. “That would be a big deal,” said Giomi, the Leiden biophysicist.
When Deamer, the membrane-first proponent, read the new paper, he recalled having once observed something like the predicted behavior in hydrocarbon droplets he had extracted from a meteorite. When he illuminated the droplets in near-ultraviolet light, they began moving and dividing. (He sent footage of the phenomenon to Jülicher.) Nonetheless, Deamer isn’t convinced of the effect’s significance. “There is no obvious way for the mechanism of division they reported to evolve into the complex process by which living cells actually divide,” he said.
Other researchers disagree, including Tang. She says that once droplets started to divide, they could easily have gained the ability to transfer genetic information, essentially divvying up a batch of protein-coding RNA or DNA into equal parcels for their daughter cells. If this genetic material coded for useful proteins that increased the rate of droplet division, natural selection would favor the behavior. Protocells, fueled by sunlight and the law of increasing entropy, would gradually have grown more complex.
Jülicher and colleagues argue that somewhere along the way, protocell droplets could have acquired membranes. Droplets naturally collect crusts of lipids that prefer to lie at the interface between the droplets and the surrounding liquid. Somehow, genes might have started coding for these membranes as a kind of protection. When this idea was put to Deamer, he said, “I can go along with that,” noting that he would define protocells as the first droplets that had membranes.
The primordial plotline hinges, of course, on the outcome of future experiments, which will determine how robust and relevant the predicted droplet division mechanism really is. Can chemicals be found with the right two states, A and B, to bear out the theory? If so, then a viable path from nonlife to life starts to come into focus.
The luckiest part of the whole process, in Jülicher’s opinion, was not that droplets turned into cells, but that the first droplet — our globule ancestor — formed to begin with. Droplets require a lot of chemical material to spontaneously arise or “nucleate,” and it’s unclear how so many of the right complex macromolecules could have accumulated in the primordial soup to make it happen. But then again, Jülicher said, there was a lot of soup, and it was stewing for eons.
“It’s a very rare event. You have to wait a long time for it to happen,” he said. “And once it happens, then the next things happen more easily, and more systematically.”
See the full article here .
Please help promote STEM in your local schools.
Formerly known as Simons Science News, Quanta Magazine is an editorially independent online publication launched by the Simons Foundation to enhance public understanding of science. Why Quanta? Albert Einstein called photons “quanta of light.” Our goal is to “illuminate science.” At Quanta Magazine, scientific accuracy is every bit as important as telling a good story. All of our articles are meticulously researched, reported, edited, copy-edited and fact-checked.
